Prakash
Interviews
AgBioWorld
Articles
Other
Articles
Biotech
and Religion
Media Contacts
Press
Releases
Special
Topics
Spanish
Articles
|
 |
 |
 |
Experience from the Humanitarian Golden Rice Project: Extreme Precautionary
Regulation Prevents Use of Green Biotechnology in Public Projects
BioVision Alexandria 3-6 April 2004
By Ingo Potrykus
Professor emeritus Plant Sciences, ETH Zuerich, Switzerland

Biofortification can complement traditional interventions against
malnutrition.
In developing countries 500,000 per year become blind and up to 6,000
per day die from vitamin A-malnutrition. And this is despite enormous
efforts from public and philanthropic institutions to reduce this medical
problem with the help of traditional interventions such as supplementation,
fortification, encouragement for diet diversification, etc. This heavy
toll poor people in developing countries are paying to vitamin A-malnutrition
will continue year by year, if we do not find a way to complement traditional
interventions by sustainable and unconventional ones. One of those could
be based on nutritional improvement of basic staple crops via "bio-fortification"
- genetic improvement with regards to micronutrients and vitamins. Plant
breeding and genetic engineering offer two complementing approaches.
The major micronutrient deficiencies concern iron, zinc, and vitamin A.
Vitamin A-deficiency is wide-spread amongst rice-depending poor because
rice does not contain any pro-vitamin A (Plants do not produce vitamin
A but pro-vitamin A (carotenoids), which our body converts into vitamin
A) . Dependence on rice as the predominant food source, therefore, necessarily
leads to vitamin A-deficiency if poverty prevents a diversified diet,
most severely affecting children and pregnant women. The medical consequences
for the vitamin A-deficient 400 million rice-consuming poor are severe:
impaired vision - in the extreme case irreversible blindness - impaired
epithelial integrity against infections, reduced immune response, heamopoieses,
skelettal growth, etc. Rice containing pro-vitamin A could substantially
reduce the problem, but "bio-fortification" of rice for pro-vitamin
A is not possible without genetic engineering. The transgenic concept,
therefore, was based on the idea to introduce all genes necessary to activate
the biochemical pathway leading to synthesis and accumulation of pro-vitamin
A in the endosperm (the starch storage tissue of the seed).
The scientific breakthrough with pro-vitamin A.
"Golden Rice" contains the genes necessary to activate the
biochemical pathway for pro-vitamin A. This pathway is activated exclusively
in the endosperm. The intensity of the "golden colour" represents
the concentration of pro-vitamin A. There are different lines with different
concentrations. We aim at concentrations, where a daily diet of 200g of
rice will provide enough pro-vitamin A to substantially reduce vitamin
A deficiency. The concentration required for this purpose can only be
determined, when data from bio-availability studies are available. Experiments
on these lines are in progress, but will take until end of 2005. So far
we are working with lines in which -theoretically - the concentration
is high enough for our goal.

.
The novel trait has been transferred into several Indica rice varieties
- especially IR 64, the most popular rice variety of Southeast Asia, and
"regulatory clean" events have been selected to facilitate the
processing through the deregulatory process. (Ye, X., Al-Babili, S.,
Klöti, A., Zhang, J., Lucca, P., Beyer, P., Potrykus, I. (2000).
Engineering provitamin A ( -carotene) biosynthetic pathway into (carotenoid-free)
rice endosperm. Science 287, 303-305. Beyer P, Al-Babili S, Ye X, Lucca
P, Schaub P, Welsch R, Potrykus I (2002) Golden Rice: introducing the
-carotene biosynthetic pathway into rice endosperm by genetic engineering
to defeat vitamin A deficiency. J Nutrition 132: 506S-510S. Tran Thi Cuc
Hoa, Salim AlBabili, Patrick Schaub, Ingo Potrykus, and Peter Beyer (2003).
Golden Indica and Japonica rice lines amenable to deregulation. Plant
Physiology 133, 161-169.)
Golden Rice will be made freely available in a humanitarian project.
Golden Rice will be made available to developing countries in the framework
of a "Humanitarian Golden Rice Project". This was from the beginning
a public research project, designed to reduce malnutrition in developing
countries. Thanks to strong support from the private sector and donations
of "free licences for humanitarian use" for intellectual property
rights involved in the basic technology, the hurdle of extended IPR linked
to the technology used in the scientific project could be overcome. This
enables us to collaborate with public rice research institutions in developing
countries on the basis of "freedom-to-operate" towards the development
of locally adapted Golden Rice varieties. Once Golden Rice varieties have
passed the national bio-safety procedures, it will be made available to
subsistence farmers free of charge and limitations. It will become their
property and they can - year after year - use part of their harvest for
the next sowing (without paying anything to anybody). The farmers will
use their traditional farming systems and they will not require any additional
agronomic inputs. Therefore, there will be no "new dependencies"
from anyone. And there is no conceivable risk to any environment which
would justify not to grow Golden Rice in the field for breeding and up-scaling
reasons. This progress since the scientific breakthrough in 1999 was possible
thanks to a novel type of "public-private-partnership". Thanks
to an agreement with Syngenta and other agbiotech industries, the use
of Golden Rice is free of licenses for "humanitarian use", defined
as "income from Golden Rice per year and farmer below $ 10'000.-.
"Commercial use", however, (above $ 10'000.- per year) requires
a license from Syngenta. Humanitarian use is based on (license-free) sublicenses
from the Humanitarian Golden Rice Board (contact: Professor Ingo Potrykus)
to public rice research institutions. This sublicense agreement ensures
that the material is handled according to established GMO rules and regulations,
and that the target population - subsistence farmer and urban poor -receive
the material without any additional cost for the trait.
Locally adapted varieties are being developed in national institutions
in the framework of a Humanitarian Golden Rice Network under the guidance
of a Humanitarian Golden Rice Board.
Development of locally adapted Golden Rice varieties as well as application
to national bio-regulatory authorities for field testing and deregulation
is in the hands of national and international public rice research institutions.
To date this "Humanitarian Golden Rice Network" includes 16
such institutions in Bangladesh, China, India, Indonesia, South Africa,
The Philippines, and Vietnam. The network is under the strategic guidance
of the Humanitarian Golden Rice Board, and under the management of a network
coordinator with office at the International Rice Research Institute (IRRI),
Philippines. The Humanitarian Board has, so far, no legal status and benefits
from the expertise of international authorities such as Dr. Gurdev Khush,
retired from IRRI (rice breeding), Prof. Robert Russell, Laboratory for
Human Nutrition, Tufts University Boston (vitamin A-malnutrition), Dr.
Howarth Bouis, International Food Policy Research Institute (IFPRI) Washington
(bio-fortification), Dr. Gary Toenniessen, The Rockefeller Foundation
New York (food security in developing countries), Dr. Robert Bertram,
USAid Washington (development in Third World agriculture), Dr. Adrian
Dubock, Syngenta (product development and intellectual property rights),
Dr. Ren Wang / Dr. William Padolina IRRI (international cooperation in
rice research), Professor Peter Beyer (co-inventor) university of Freiburg
(scientific progress underlying bio-fortification in pro-vitamin A and
other micronutrients), Dr. Katharina Jenny, Swiss Development Cooperation
Bern (technology transfer and trans-sectorial issues), and Professor Ingo
Potrykus (co-inventor), retired from ETH Zuerich, chairman (public relations
and information).
Biofortified seeds have an unmet potential for sustained solutions.
Bio-fortification (complementation for missing micro-nutrients with the
help of genetic complementation) of the basic staple crops for poor populations
in developing countries is, most probably, the most sustainable and cost-effective
approach to reduction in micro-nutrient malnutrition. (For more information
on the concept of bio-fortification and a recent challenge program of
the CGIAR see homepage www.harvestplus.org). Golden Rice represents the
first example of bio-fortification achieved via genetic engineering. Research
investment for this trait (bio-fortification for pro-vitamin A) was relatively
modest ($ 2,4 million over 9 years) and financed from funds for basic
research.
Product development, however, from this scientific brake-through is time
consuming and requires additional funding, but again one-time only for
each event. Expenses are increasing really dramatically when working through
the bio-safety assessments required for deregulation. But again this is
a one-time investment. As soon as a novel bio-fortified variety is deregulated
and can be handed out to the farmer, the system demonstrates its unique
potential, because from this point on, the technology is built into each
and every seed and does not require any additional investment, for an
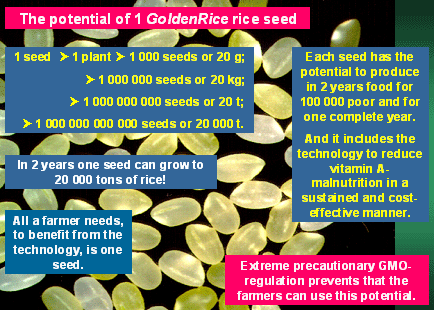
unlimited period of time. Just consider the potential of a single Golden
Rice seed: Put into soil it will grow to a plant which produces, at least,
1 000 seeds; a repitition will yield at least 1 000 000 seeds; next generation
produces already 1 000 000 000 seeds and in the fourth generation we arrive
at 1 000 000 000 000 seeds. These are 20 000 metric tons of rice and it
takes only two years to produce them. From these 20 000 tons of rice 100'
000 poor can survive for one year, and if they use Golden Rice they have
an automatic vitamin A supplementation reducing their vitamin A-malnutrition,
and this protection is cost-free and sustainable. All a farmer needs to
benefit from the technology is one seed! There is no additional input
required compared to "normal rice". And for urban poor there
is no premium on vitamin A-rice. There are enough seeds to be handed out
to many farmers, but this can not be done, because Golden Rice is a "GMO"
(genetically modified organism) and those are highly regulated. And the
Humanitarian Golden Rice Board has decided to follow the established rules
and regulations.
Extreme precautionary regulation, however, prevents use so far and
ignores the potential benefits.
Considering the history of Golden Rice (the technology is often considered
risky because it is so fast!) it took 10 years (from 1980 to 1990) to
develop the necessary technology of placing genes into rice. It took further
9 years (from 1990 to 1999) to introduce the genes required to establish
the biochemical pathway leading to pro-vitamin A in the seed. And it took
further 5 years (from 1999 to 2004) to develop a Golden Rice "product"
and carry it across a series of GMO-specific hurdles such as IPRs. And
it will take, probably, at least 5 more years to advance the first Golden
Rice product through the deregulatory procedure: Therefore, it took 30
years if we include technology development, and it took still 20 years
for the single specific case. Considering that Golden Rice could substantially
reduce blindness (500 000 per year) and death (2-3 million per year) 20
years are a very long time period, and I do not think that anyone should
complain that this was "to fast"! If it were possible to shorten
the time from science to the deregulated product, we could prevent blindness
for hundreds of thousands of children! However, the next 5 years will
have to be spent on the required "bio-safety assessments" to
guarantee that there is no putative harm from Golden Rice for the environment
and the consumer. Nothing speaks against a cautious approach, but present
regulatory praxis follows an extreme interpretation of the "precautionary
principle" with the understanding that not even the slightest hypothetical
risks can be accepted or left untested, and at the same time all putative
benefits are totally ignored. Looking at Golden Rice and the problem of
environmental risk assessment discloses how irrational the present system
operates : The author has, over the last four years, not found any ecologist,
including those from the "professional GMO-opposition", who
could construct a half-way realistic hypothetical risk from Golden Rice
to any agronomic or wild environment. This is not surprising because the
entire biology of the system - low amounts of additional -carotene in
the endosperm in plants which are loaded with -carotene in every organ
except for the root - does not provide for any selective advantage in
any environment, and therefore can not pose any substantial risk. Despite
this fact Golden Rice is still awaiting the first permission for the first
small-scale field release, in which environmental risks have to be studied
experimentally! So far to the "risk" side of the equation. And
the "benefit"? Golden Rice could prevent blindness and death
of hundreds of thousands of children but can not do so, so far, because
risk assessment notoriously is ignoring a risk-benefit analysis!
Present Deregulation is extremely demanding on time and financial
resources
What then is required for the deregulatory procedure? First of all, it
is advisable to focus on one carefully selected transgenic event, which
is as "regulatory clean" as possible - that is, it must not
include characters which are a priori unpopular with regulatory authorities,
such as "multiple integrations", "rearrangements",
"read-through across T-DNA borders", "microbial origins
of replication", "ballast DNA", etc. This requires the
production of many hundreds of similar transgenic events with the same
DNA construct. This construct itself must have been assembled taking into
account the requirements of the regulatory authorities in the later deregulation
process. Only when working on the basis of a "regulatory clean construct"
and with "regulatory clean transformation technology" there
is a chance to survive the deregulation. Such a carefully selected event
can then be used to start the series of bio-safety assessment experiments
traditionally expected to prove or disprove any putative bio-safety hazard.
(It is a waste of time to enter the process with material which is not
"regulatory clean" at the onset). The consequence of this approach
is, that nearly 99% of transgenic events, and often those with the highest
levels of expression, have to be discarded. Already this first step of
mass production of many hundreds of similar events and subsequent destruction
of most is beyond the scope of any public research institution, not only
in developing but also in developed countries. No funding agency would
be willing to finance this step. This is, however, the first prerequisite
for entering the deregulation procedure with some chance for success.
Once the right material is ready, bio-safety assessment can start. There
are "event-independent" studies which refer to the introduced
genes and their function in general, and which are valid for all events
produced with these genes. "Exposure evaluation" (for the novel
trait, e.g. pro-vitamin A in rice) studies the intended use and bio-availability.
This study alone takes about 3 years, because the material has to be produced
in specific plant growth chambers due to the lack of permission for field
release (see above!). "Protein production and equivalence" analyses
the proteins through which the genes fulfil their function. For this purpose
the proteins have to be isolated from the plant, biochemically characterized,
and their function confirmed. Lack of homology to toxins and allergens,
rapid degradation in gastric/intestinal studies, heat lability, acute
toxicity in rodent feeding, screening for further putative allergens and
toxins are assumed to ensure that no unintended toxin or allergen will
be consumed with Golden Rice. This seems reasonable if we ignore that
most people have eaten these genes and gene-products throughout his/her
life from other food sources. To study, as has been proposed, whether
Daffodil toxins have been introduced into Golden Rice (one gene is from
Daffodil and it is not advisable to consume Daffodil ) demonstrates how
far an assessment can be from science: what has been transferred is one
defined piece of DNA with no relation whatsoever to any toxin or allergen!).
These studies take at least 2 years of intensive work in a well equipped
biochemistry laboratory. What has been described, so far, was only an
introduction; the real work comes with the "event-dependent"
studies: "Molecular characterization and genetic stability"
(single copy effect; marker gene at same locus; simple integration; Mendelian
inheritance over at least three generations; no potential gene disruption;
no unknown open reading frames; no DNA transfer beyond borders; no antibiotic
resistance gene or origin of replication; insert limited to the minimum
necessary; insert plus flanking regions sequenced; phenotypic evidence
and biochemical evidence for stability over three generations). "Expression
profiling" (Gene expression levels at key growth stages; evidence
for seed-specific expression); "Phenotype analysis" (Field performance,
typical agronomic traits, yield compared to isogenic lines; pest and disease
status same as origin). "Compositional analysis" (Data from
two seasons times six locations times three replications on proximates,
macro- and micro-nutrients, anti-nutrients, toxins, allergens; data generated
on modified and isogenic background). "Environmental risk assessment".
This requires 4-5 years of an entire research team.
No public scientist or institution can afford such a deregulation
procedure.
It is rather obvious, that no scientist nor scientific institution in
the public domain has the potential, or funding, or motivation to perform
such bio-safety experiments. It is, therefore, no surprise, that virtually
all transgenic events, so far, taken through the deregulatory procedure
are (directly or indirectly) from the private sector and carry the potential
for substantial financial reward. Humanitarian projects to the benefit
of the poor obviously do not fall into this category, although the benefit
would apply to many millions. There is a lot of good intention world wide
in the public sector to exploit the potential of green biotechnology for
the benefit of the poor in developing countries. If our society, however,
continues with the present "extreme precautionary" approach
to bio-safety assessment, it is absolutely unrealistic to invest any further
funds into public research for this purpose. Of course, there would be
interesting scientific progress, but there will be no product, and especially
no product passing through regulation. And, consequently, all this work
will have no practical output and nobody in the target population would
have any benefit.
Extreme precautionary regulation is there for a number of reasons,
but none of those is justified.
Why then do we have this GMO-regulation? First of all, there are historic
reasons. At the beginning of GMO-technology development it was sensible
to be careful ("precautionary") and the scientists themselves
- at that time working not with plants but with human-pathogenic micro-organisms
- established regulations based on the notion that the consequence of
the technology could lead to "unpredictable genome alterations".
Experience, after more than 20 years with transgenic plants and their
practical application on 50 million hectares farmland as well as from
many hundreds of "biosafety" experiments in which bio-safety
questions in context with transgenic plants have been carefully studied,
led to numerous original publications and reports from academic institutions
which all come to the conclusion, that there is no specific risk associated
with the technology, which would exceed risks inherent anyhow to traditional
plant breeding or natural evolution. (For a discussion on the moral imperative
of the use of genetically modified crops in developing countries please
see Nuffield Council On Bioethics, follow-up discussion paper January
2004, homepage www.nuffieldbioethics.org) Why then do we maintain GMO
regulation and even extend it to ever more extreme precautionary regulation?
The answer to this question often follows the notion that we have to do
so to built trust in the technology for its acceptance by the consumer.
Experience with this strategy over the last 10 years, however, demonstrates
clearly that this approach did not work in Europe and many developing
countries, and this is not surprising. How should a "normal"
citizen understand that his/her government is regulating a technology
in an extreme restrictive manner, if this technology is without specific
risks. Every unbiased citizen will, of course, assume that his/her government
is taking rational decisions and the technology must be as dangerous as
the regulation implies. Consequently, maintenance of extreme precautionary
regulation builds mistrust instead of trust. Why then do we not at least
clear regulation from all scientifically unjustified and opportunistic
ballast to build a rational regulatory procedure? It seems that not many
institutions have an interest or the political power to do so. If we consider
the potential GMO technology has with regards to food security in developing
countries than numerous international organizations should have an interest,
but neither FAO, nor WHO or UNIDO will have the courage and power to do
so. What prize is our society paying for this opportunistic attitude towards
an established "extreme precautionary regulatory" system, in
function world-wide? Very clearly: GMO-technology will not reduce hunger
and malnutrition, and will not protect the environment in developing countries.
The use of the technology will be restricted to "luxury projects",
with safe financial returns, of the private sector and in developed countries.
There will, of course, be some spin-offs from these projects into developing
countries, and these may even carry some benefits for the poor - such
as "insect-resistant cotton", but there will be no product development
focussing on urgent and specific needs of the poor in developing countries,
such as "food security"!
Justification for extreme precautionary regulation ignores the basic
genetic facts of traditional crop varieties.
GMO technology has the potential to support and to complement traditional
plant breeding. In the context of the discussion on GMO-regulation, which
is justified with the argument that genetic engineering leads to "unpredictable
genome alterations" it may be helpful to remember a few basic facts
concerning all our plant-based food which is derived from crop plant varieties,
which, without any exception, have been developed through traditional
plant breeding.
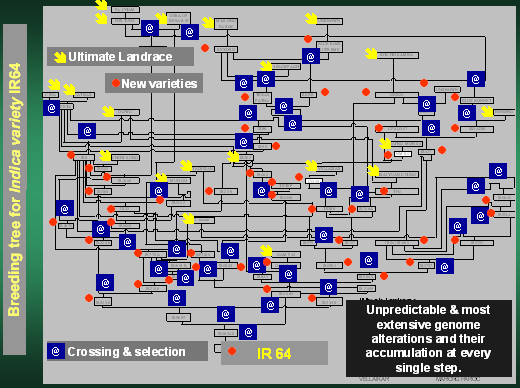
Traditional Plant breeding leads to totally unpredictable and most
severe alterations of the genome.
Plant breeding is using the technique of "crossing followed by selection"
to combine traits of agronomic and nutritional interest and to exclude
undesired traits. Starting material for this procedure are "landraces"
of crop plants, originally identified and selected by indigenous farmers.
Landraces differ from each other in traits because they differ in "mutations".
Mutations are "unpredictable genome alterations." In the course
of traditional breeding the technology adds un-deliberately and automatically
further (in parts very dramatic) "unpredictable genome alterations
such as "recombinations", "translocations", "deletions",
"inversions" etc. These "unpredictable" and "most
severe" genome alterations are accumulated at every breeding step
and each new traditionally bred variety is thus based on, and characterized
by an increasing array of such genome alterations. With the progress of
the breeding process varieties are combined with varieties, often with
related wild relatives of the crop plants, often further altered in their
genome by induced mutations. All our modern crop varieties - from which
we derive our food - have a long history and are composed of numerous
previous varieties and there is not the slightest doubt possible, that
all our traditionally bred crop varieties are most extensively "genetically
modified" by hundreds if not thousands of "unpredictable genome
alterations". This is, of course, also true for those varieties used
by organic farmers. We just do not call them "GMO's"!
Every traditionally bred modern crop variety is most intensively "genetically
modified".
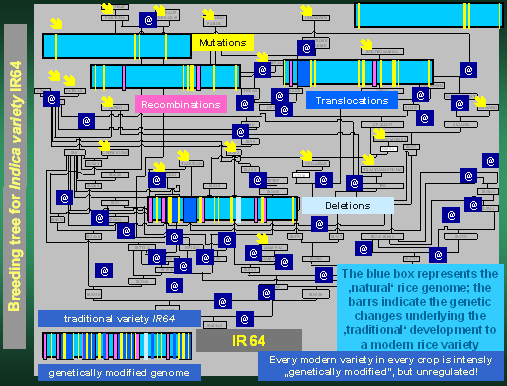
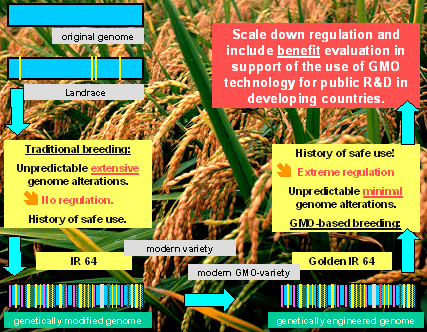
All this is exemplified in the "breeding tree" leading to IR64,
the most popular Indica rice variety, developed at the International Rice
Research Institute, Philippines and grown all over Southeast Asia. The
pictures shows graphically how intensively the original rice genome (represented
by blue boxes) has been "genetically modified" by "mutations"
(yellow bars), "recombinations" (red bars), "translocations"
(blue bars) and "deletions" (light-blue bars) to finally arrive
at the genome of IR64. Neither this variety, nor anyone of those who have
been channelled into the breeding tree have ever experienced any "bio-safety
assessment" and billions of consumers in developing countries have
consumed IR64 (as all the other rice or crop varieties) and survived on
this and the preceding varieties without any harm, and there was no unpredictable
harm to the environment as well. And this holds true for all the other
varieties in all the other crops - despite of all the "most dramatic
and unpredictable alterations to the genome"! Actually nobody could
survive without eating food from "genetically modified" crops.
"Genetically engineered" varieties differ from the "genetically
modified" ones in small, precise, similar, and well studied alterations.
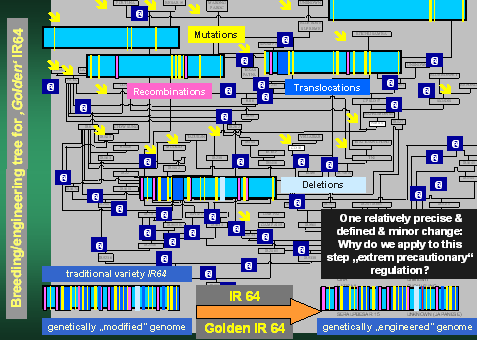
For Golden Rice we have taken this variety IR64 and added two precisely
defined genes into the 50 000 gene-genome of rice, using a technology
which is by orders of magnitude more precise than traditional breeding,
to provide pro-vitamin A in the seed to reduce vitamin A-malnutrition.
This is now an example of a "genetically engineered" variety
- a "GMO" - and such a plant is now falling under "extreme
precautionary" regulations despite the fact, that the engineering
step is, in comparison to the history of IR64 extremely small, perfectly
predictable, most detailed studied and without any greater risk to the
consumer or the environment. The reader is invited to find the difference
between IR64 and Golden IR64 in the picture above!
There is no scientific justification to treat "genetically engineered"
crops different from "genetically modified" ones.
Our experience with traditionally developed crop varieties tells us very
clearly that "unpredictable genome alterations" are not an argument
for extreme regulation. Why are they now, and beyond any logic, the key
argument for extreme regulation of "genetically engineered"
plants? The argument that genes may come from different organisms and
would never have found their way into a GMO can not be accepted as well.
We all know that genes are connected by a continuum in evolution and are
closely related and the "crossing barrier" between species is
a mechanism to advance evolution within a species, but not to prevent
introduction of genes. Why are GMO's singled out from the normal breeding
tree and treated according to the established rules and regulations of
an extreme precautionary principle, thus preventing their sensible use
to the benefit of the poor. This, to the authors understanding, is against
any logic and takes us back into the historical time period of "Middle
Ages" and before the "Age of Enlightenment". As this attitude
is singling out "green biotechnology" from nearly all other
modern technologies, it seems obvious, that there is a deliberate campaign
with a hidden political agenda.
Extreme precautionary regulation without risk-benefit analysis is
immoral and highly destructive.
What are the consequences of the extreme precautionary regulation of
green biotechnology for public research towards food security in developing
countries? There are numerous scientists and institutions in developing
countries who have the capacity, motivation, and often even funding to
work towards scientific progress in the areas of pest-, disease-, drought-,
heat-, cold-, saline-, heavy metal resistance with the potential to rescue
harvests and to expand agricultural productivity to hostile environments;
to improve photosynthetic efficiency and to enhance the exploitation of
natural resources to increase productivity; to enhance nutritional content
to reduce malnutrition with regards to micro-nutrients such as vitamin
A etc. Very few of those, however, have the financial and mental capacity
to transform a scientific success into an applicable "product",
which is the first prerequisite for benefit of the poor from a scientific
advance. Probably no scientist nor institution in the public domain, however,
have the resources, experience, and determination to carry a single GMO
product across the hurdles of to days extreme precautionary regulatory
procedures. Regulatory authorities in developing countries are less experienced,
more insecure, and therefore, more stringent than their colleagues in
developed countries. Even with support from the experienced private sector
deregulation of a novel GMO product has become a gigantic task. It is,
therefore, very obvious that, if we continue with the present regulatory
standards, the potential of green biotechnology will not reach the poor.
In the 19th century a cultural taboo let to the tragic death of an
18 year old princes.

.
In the 21st century ignorance of our society leads to avoidable misery
and death of millions.

"Genetically engineered" plants are not unusual plants, filled
with mysterious dangers for the consumer and the environment. Europe can
be proud of its cultural heritage of the "Age of Enlightenment"
and should rather listen to the advice of science than that of "witch
hunters". It is Europe's responsibility to help developing countries
to harness the potential of green biotechnology, however the European
attitude badly affects the attitude in developing countries.
Europe can afford such an extreme negative attitude because it can buy
whatever it wants on the world market. However, for developing countries
such an attitude leads to unnecessary death and misery of many millions.
Let me close with two citations from the follow-up discussion paper of
the Nuffield Council on Bioethics 2004: "The European Union is ignoring
a "moral imperative" to promote genetically modified crops for
their great potential for helping the developing world." "We
believe EU regulators have not paid enough attention to the impact of
EU regulations on agriculture in developing countries." Our societies
have wasted too much time in a long phase of "risk-obsession".
Stop following the "wrong prophets"! Putative benefits by far
outdate the "phantom risks". It is time to return to "common
sense".
Ingo Potrykus, PhD, Professor emeritus Plant Sciences,
Swiss Federal Institute of Technology (ETH), Zuerich, Switzerland
ingo@potrykus.ch
|

